Licensed Pharmacist
Insight Connect Solutions Inc.
Descrição do Trabalho
Benefícios
Benefícios gerenciados pelo Governo
Pagamento no décimo terceiro mês, Empréstimo para Funcionários, Fundo Pag-Ibig, Saúde, SSS/GSIS
Desenvolvimento Profissional
Treinamento de Trabalho
Tempo de desligamento e Saída
Maternidade e licença de paternidade, Licença médica, Licença Pai Solo, Férias Deixadas
Balanço de Vida
Trabalho de casa
Regulatory Compliance:
- Ensure company operations and products comply with the rules and regulations of the Philippine FDA. -
- Monitor updates and advisories issued by the FDA and ensure immediate company-wide implementation.
- Prepare and submit compliance reports and documentation to the FDA.
Licensing and Registration
- Oversee the application and renewal of FDA licenses such as LTO (License to Operate), CPR (Certificate of Product Registration), and other necessary permits for pharmaceuticals, cosmetics, food, and medical devices. - Maintain up-to-date records of all regulatory filings and certificates.
Audit and Inspection Handling:
- Prepare the company for routine and spot inspections by FDA officers. - Serve as the point person during FDA audits and respond to FDA findings or notices
Documentation and Record Keeping
- Maintain comprehensive and accurate documentation of all compliance-related activities. - Ensure traceability and document integrity for all FDA-regulated products.
Internal Training and Policy Development
- Educate staff and departments on FDA regulations and compliance protocols.
- Develop internal SOPs (Standard Operating Procedures) based on regulatory guidelines.
Adverse Event and Recall Management
- Monitor and report adverse drug reactions or product complaints to the FDA as required.
- Coordinate product recalls in case of FDA-mandated withdrawals or safety issues.
Liaison with Regulatory Authorities:
- Communicate effectively with FDA personnel and other government agencies. - Ensure timely submission of queries, appeals, and supporting documents.
- 2-3 years experience with FDA & BAI regulatory application
- Fresh Graduates are welcome to apply
- Licensed Pharmacist required
- Good communication skills
- Must be willing to work on-site at BGC Taguig
- Can start ASAP
MJ de Belen
HR/Admin ExecutiveInsight Connect Solutions Inc.
Responder Hoje 0 Vezes
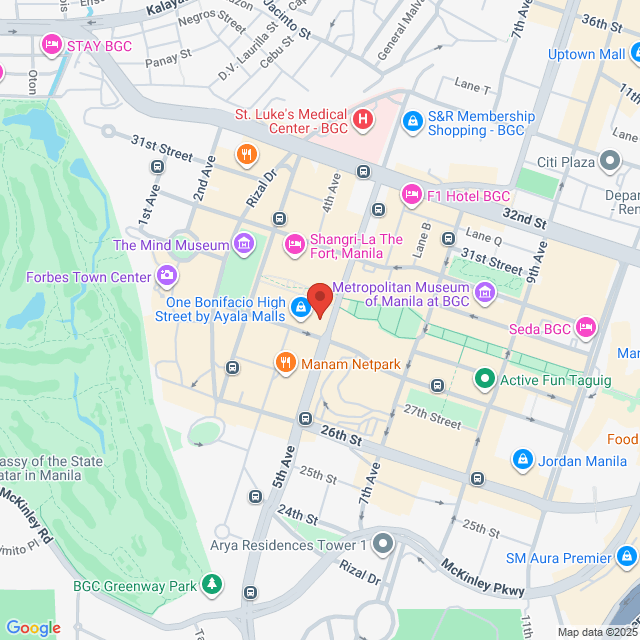
Local de trabalho
24th, The Philippine Stock Exchange, Inc.. PSE Tower, 5th Avenue corner 28th Street, BGC 1634, Taguig, Philippines
Postado em 02 July 2025
Regulatory Officer
 Kareila Management Corporation
Kareila Management CorporationR$2.3-3.2K[Mensual]
No - Taguig1-3 Anos ExpBacharelTempo Inteiro

Cris Quinzanos,CRSPTalent Acquisition Specialist
Licensed Pharmacist
 MedExpress Pharmacy
MedExpress PharmacyR$1.8-2.3K[Mensual]
No - Cidade do QuezonGraduação/Aluno FrescoBacharelTempo Inteiro

MedExpress RecruitmentHR Officer
Staff Pharmacist
 University of Santo Tomas Hospital
University of Santo Tomas HospitalR$1.9K[Mensual]
No - Manila<1 ano de experiênciaBacharelTempo Inteiro

Regine Veronica ReginoHR Assistant - Recruitment
Licensed Pharmacist
 PNI Business Solutions Inc.
PNI Business Solutions Inc.R$2.8-3.2K[Mensual]
No - Pasig1-3 Anos ExpBacharelTempo Inteiro

Yza PNIRecruitment Specialist
Licensed Pharmacist
 VBP Dermatology Institute Inc - Makati City
VBP Dermatology Institute Inc - Makati CityR$1.8-2.3K[Mensual]
No - MakatiGraduação/Aluno FrescoBacharelTempo Inteiro
HR RecruitmentHR Officer
Sign In to Chat with Boss
Bossjob Safety Reminder
If the position requires you to work overseas, please be vigilant and beware of fraud.
If you encounter an employer who has the following actions during your job search, please report it immediately
- withholds your ID,
- requires you to provide a guarantee or collects property,
- forces you to invest or raise funds,
- collects illicit benefits,
- or other illegal situations.

