Logistic Specialist
101 Supply Chain Solutions Inc.
Descrição do Trabalho
- Ensures compliance and assist in the implementation of Quality Management System (QMS), compliance to regulatory requirements, and applicable Standards (e.g. GMP, HACCP, FSSC, etc.)
- Initiate and implement quality initiatives and programs for continual improvement
- Provides guidance to operational areas (e.g. warehouse) to ensure robust quality system
- Effectively review/approve documented procedures (e.g. SOPs, Work Instructions) to ensure quality assurance attributes are met
- Ensures proper documentation and archiving of QMS documents, along with maintaining traceability
- Act as the Qualified Person in compliance to FDA regulations (e.g. license-to-operate)
- Attends during regulatory inspections and customer audits
- Develop, revise, and maintain Quality Assurance policies, programs, and procedures
- Conducts training of Quality Assurance related policies, programs, and procedures
- Performs quality related tasks including but not limited to processing, reviewing, and documenting change control, complaints, CAPA, internal audit, and management review
- Ensures that product and/or process deviations, non-conformities, complaints, recalls are reported, investigated, resolved, and documented properly
- Develop, review, and conduct Risk Management Plan and Risk Assessment activities
- Review/approve validation projects, plans, protocols, and reports
- Perform quality activities related to Supplier Management such as vendor assessment and audits, as necessary
- At least a bachelor’s degree in chemistry, chemical engineering, food technology, food and nutrition
- Preferably licensed/registered
- Preferably 1-2 years of experience in related field
- Experience in 3rd -party logistics (3PL) handling regulated products such as food, cosmetics, etc.
- In-depth knowledge of Quality Management System and Regulatory Requirements (e.g. FDA, GMP, HACCP, FSSC etc.)
- Computer literate with basic knowledge in MS Office
- Exhibit attention to detail in all areas of work
- Display excellent and effective communication skills, both verbal and written
- Display effective and efficient data collection, management, problem analysis, and problem-solving skills
- Planning and organizing tasks
- Exhibit leadership and think strategically
- Maintain a positive attitude and intrinsic motivation when solving problems
- Be productive, organized, and able to manage deadlines
- Has the ability to adjust to changing regulations, new products, new process, and/or technologies
- Has the ability to prioritize projects appropriately, work with complex timelines, and meet department objectives
- Has the ability to manage multiple projects while maintaining high quality outcomes
- Ability to work proactively with workmates, both superiors and subordinates
WENEFREDO NAAGAS
CEO101 Supply Chain Solutions Inc.
Responder Hoje 1 Vez
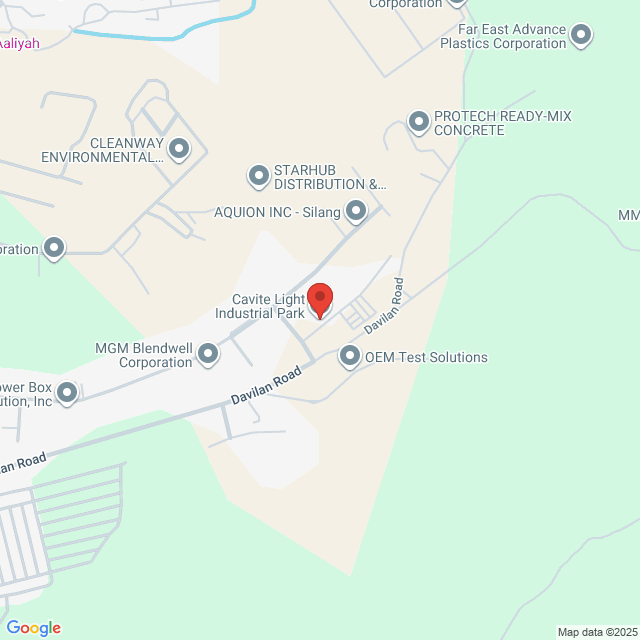
Local de trabalho
Blk 8 Lot 8, Cavite Light Industrial Park. 7253+4W3, Mallorca City Avenue Brgy, Silang, Cavite, Philippines
Postado em 18 August 2025
Shift Head, Quality Assurance Analyst
 Cavite Biofuel Producers Inc.
Cavite Biofuel Producers Inc.R$2.7-3.2K[Mensual]
No - Cavita1-3 Anos ExpBacharelTempo Inteiro

Hazzle Anne De la VegaHR Specialist (Training & Recruitment)
QC Asst. Supervisor
 Sanno Philippines Manufacturing Corporation
Sanno Philippines Manufacturing CorporationR$1.8-2.3K[Mensual]
No - Cavita1-3 Anos ExpBacharelTempo Inteiro
admin-sanno admin2Recruiter
QC/QA Supervisor
 Philippine Toei Chemical Corporation
Philippine Toei Chemical CorporationR$3.2-4.1K[Mensual]
No - Cavita1-3 Anos ExpBacharelTempo Inteiro

ENERLYN ENRIQUEZHR Officer
QA Specialist
 Cavite Meats and Agri Trading Inc.
Cavite Meats and Agri Trading Inc.R$1.4-1.8K[Mensual]
No - CavitaGraduação/Aluno FrescoBacharelTempo Inteiro

trainingdevelopmentRecruiter
QA Specialist
 101 Supply Chain Solutions Inc.
101 Supply Chain Solutions Inc.R$1.4-1.8K[Mensual]
No - Cavita1-3 Anos ExpBacharelTempo Inteiro

WENEFREDO NAAGASCEO
Sign In to Chat with Boss
Bossjob Safety Reminder
If the position requires you to work overseas, please be vigilant and beware of fraud.
If you encounter an employer who has the following actions during your job search, please report it immediately
- withholds your ID,
- requires you to provide a guarantee or collects property,
- forces you to invest or raise funds,
- collects illicit benefits,
- or other illegal situations.
