Regulatory Affairs Pharmacist Manager - 5 yrs Experience
Dempsey Resource Management Inc.
Descrição do Trabalho
Benefícios
Recompensas e reconhecimento de funcionário
Bônus de desempenho
Benefícios gerenciados pelo Governo
Pagamento no décimo terceiro mês, Empréstimo para Funcionários, Fundo Pag-Ibig, Feriados Pagos, Saúde, SSS/GSIS
Tempo de desligamento e Saída
Licença parental, Licença médica, Férias Deixadas
Compliance and Control:
- Register our cosmetics, drugs, food supplements, medical device and household hazardous product at FDA.
- Prepare for initial or renewal of LTO licenses and coordinate with the FDA inspector
- Handle and resolve Notice of Compliance from FDA
- Maintain current knowledge of relevant regulations in Cosmetics and keeping up to date with the changes in regulatory legislation and guidelines.
- Ensures that formulation confirms to Philippine FDA standards and is within the approved limit.
- Ensures that assigned warehouses are compliant with FDA requirements.
- Conducts product test for all finished goods distributed and imported by the company.
- Schedules and facilitate monthly warehouse visit and coordinate the schedule of pest control
Customer Relations:
- Assist in updating related departments and the management on latest regulatory requirements and FDA advisories.
- Conducts product test and investigate product complaints.
- Assists in answering customer inquiries and concerns.
Suppliers:
- Assigned to handle products from imported and local suppliers.
- Conducts annual suppliers and toll manufacturer audits.
- Coordinates with manufacturers for Product Information File for filing based on ASEAN Cosmetics Directive.
- Process initial and renewal of memorandum of agreements from manufacturers.
- Request for updated GMP and ISO from manufacturers.
Requirements:
- Bachelor's degree in Pharmacy or related field.
- Experience in regulatory affairs within the pharmaceutical industry.
- Strong knowledge of FDA, EMA, and other regulatory bodies.
- Excellent written and verbal communication skills.
- Attention to detail and strong organizational skills.
- Ability to work independently and as part of a team.
- Proficiency in regulatory submission software and databases.
- Understanding of clinical trial regulations and processes.
1. Expected salary: Regulatory Manager : 40-45K
2. Work onsite : Caloocan
3. Work Monday to Friday 8:30am - 5:30pm
4. Need to know if He or She has experience in LTO inspection and release of CPR
5. Availability to Report
Aisa Refugia
Dempsey Resource Management Inc.
Mais de dez respostas hoje
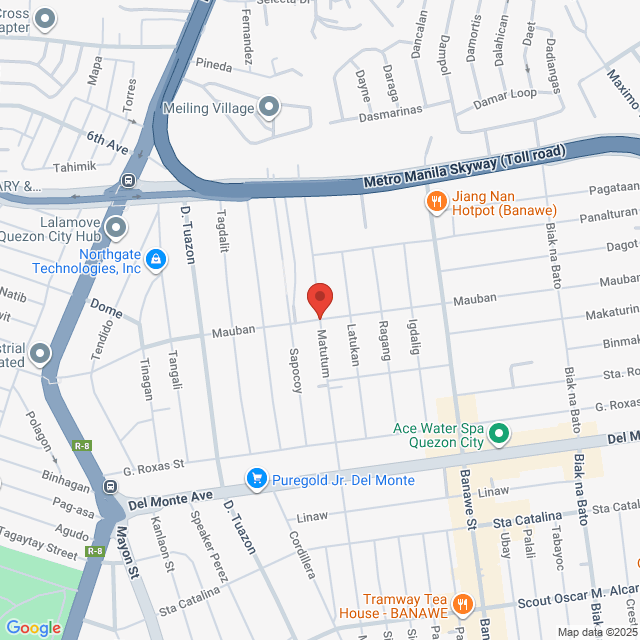
Local de trabalho
Barangay San Jose , Quezon City. 71 Mauban, Quezon City, Kalakhang Maynila, Philippines
Postado em 20 October 2025
Regulatory Pharmacist
 Main Source Manufacturing and Trading Corp.
Main Source Manufacturing and Trading Corp.R$1.8-2.3K[Mensual]
No - Caloocan1-3 Anos ExpBacharelTempo Inteiro

MAIN SOURCE MANUFACTURING AND TRADING CORPHR Manager
Regulatory Pharmacist
 Lifestrong Marketing Inc.
Lifestrong Marketing Inc.R$2.3-2.7K[Mensual]
No - Caloocan<1 ano de experiênciaBacharelTempo Inteiro

Angel Joy CauilanTalent Acquisition Associate
Regulatory Affairs Pharmacist Associate - 2 years Experience
 Dempsey Resource Management Inc.
Dempsey Resource Management Inc.R$2.3-2.6K[Mensual]
No - Caloocan1-3 Anos ExpBacharelTempo Inteiro
Aisa Refugia
Regulatory Affairs Pharmacist
 Allegens Healthcare Philippines Inc.
Allegens Healthcare Philippines Inc.Negociável
No - Makati1-3 Anos ExpBacharelTempo Inteiro

Nguyen Tuyet NuHR Manager
Regulatory Pharmacist
 Ambica International Corporation
Ambica International CorporationR$1.8-2.7K[Mensual]
No - PasaySem necessidade ExpBacharelTempo Inteiro
Race SeldaRecruitment Supervisor
Sign In to Chat with Boss
Bossjob Safety Reminder
If the position requires you to work overseas, please be vigilant and beware of fraud.
If you encounter an employer who has the following actions during your job search, please report it immediately
- withholds your ID,
- requires you to provide a guarantee or collects property,
- forces you to invest or raise funds,
- collects illicit benefits,
- or other illegal situations.
