Regulatory Pharmacist
Main Source Manufacturing and Trading Corp.
Descrição do Trabalho
Benefícios
Benefícios gerenciados pelo Governo
Pagamento no décimo terceiro mês, Fundo Pag-Ibig, Feriados Pagos, Saúde, SSS/GSIS
Saúde e Bem-Estar
HMO
Benefícios de Habilidades
Desconto do funcionário
Tempo de desligamento e Saída
Licença médica, Licença Pai Solo, Férias Deixadas
- Acts as a liaison of the company and transacts with relevant government and regulatory agencies, including but not limited to the Food and Drug Administration, Bureau of Animal Industry, and Department of Trade and Industry.
- Keeps the company updated with significant issuances and requirements from different agencies.
- Guides the company in complying with Good Manufacturing Practices (GMP) and checks their implementation at work.
- Ensures the documentation control of regulatory and manufacturing documents.
- Ensures the validity of permits, licenses, and product registrations.
- Updates the company with significant issuances from regulatory agencies.
- Coordinates with regulatory agencies regarding applications and other concerns.
- Prepares the requirements for applications for permits, licenses, and product registrations.
- Discusses the completion of requirements (e.g., third-party analysis, studies, etc.) for applications of permits, licenses, and product registrations with the Plant Manager.
- Ensures that Good Manufacturing Practices are being followed during work.
- Trains company employees on the different modules of Good Manufacturing Practices.
- Coordinates plans for improvement on GMP-related matters and documentation control with the Plant Manager.
- Oversees documentation control on regulatory and manufacturing-related files.
- Writes Standard Operating Procedures relevant to Good Manufacturing Practices.
- Keeps documents up to date and relevant.
- Summarizes the forms being used in the manufacturing plant.
- Revises forms whenever changes are necessary.
- Performs and assists in quality control and quality assurance tasks when deemed necessary.
- Acts as the authorized person for the release of finished products.
- Maintains a hazard-free working environment.
- Performs other duties as may arise from time to time and as may be assigned to the employee.
MAIN SOURCE MANUFACTURING AND TRADING CORP
HR ManagerMain Source Manufacturing and Trading Corp.
Responder Hoje 1 Vez
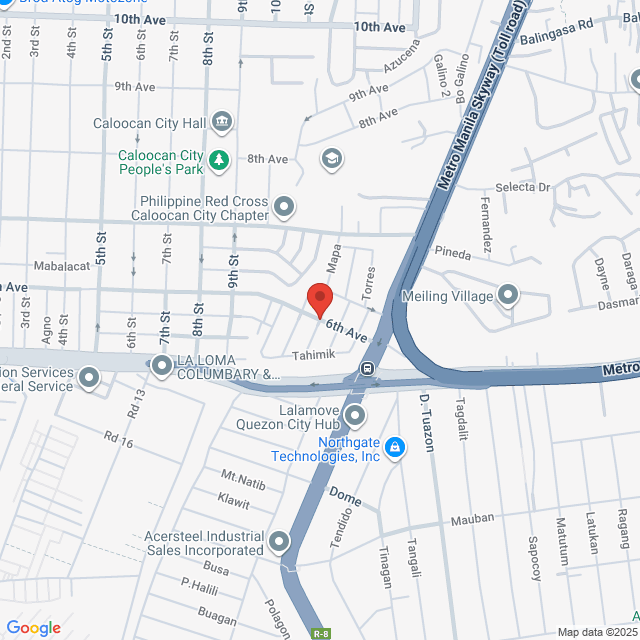
Local de trabalho
103 tahimik st, 6th Avenue & V. Mapa. 6th Avenue & V. Mapa, Grace Park East, Caloocan, Metro Manila, Philippines
Postado em 23 October 2025
Regulatory Pharmacist
 Lifestrong Marketing Inc.
Lifestrong Marketing Inc.R$2.3-2.7K[Mensual]
No - Caloocan<1 ano de experiênciaBacharelTempo Inteiro

Angel Joy CauilanTalent Acquisition Associate
Regulatory Affairs Pharmacist Manager - 5 yrs Experience
 Dempsey Resource Management Inc.
Dempsey Resource Management Inc.R$3.7-4.1K[Mensual]
No - Caloocan5-10 anos ExpBacharelTempo Inteiro
Aisa Refugia
Regulatory Affairs Pharmacist
 Allegens Healthcare Philippines Inc.
Allegens Healthcare Philippines Inc.Negociável
No - Makati1-3 Anos ExpBacharelTempo Inteiro

Nguyen Tuyet NuHR Manager
Regulatory Pharmacist
 Ambica International Corporation
Ambica International CorporationR$1.8-2.7K[Mensual]
No - PasaySem necessidade ExpBacharelTempo Inteiro
Race SeldaRecruitment Supervisor
Regulatory Pharmacist
 Raya Regenerative and Preventive Medicine
Raya Regenerative and Preventive MedicineR$2.7-3.7K[Mensual]
No - Makati<1 ano de experiênciaBacharelTempo Inteiro

Justine GamboaHR Associate

Main Source Manufacturing and Trading Corp.
51-100 funcionários
Suprimentos e Serviços de Fabricação e Industrial
View jobs hiring
Sign In to Chat with Boss
Bossjob Safety Reminder
If the position requires you to work overseas, please be vigilant and beware of fraud.
If you encounter an employer who has the following actions during your job search, please report it immediately
- withholds your ID,
- requires you to provide a guarantee or collects property,
- forces you to invest or raise funds,
- collects illicit benefits,
- or other illegal situations.
